What is an Atom?
An atom is the smallest particle into which an element can be divided without losing its chemical identity. Atoms consist of a heavy central nucleus surrounded by a cloud of negatively charged particles called electrons. The nucleus contains positive particles (protons) and electrically neutral particles (neutrons). The number of protons is called the atomic number. This number uniquely identifies each chemical element. In turn, protons and neutrons are composed of quarks.
An element is a chemical substance that is made up of single kind of atom. Iron, carbon, and hydrogen are all elements. A molecule is formed when two or more atoms of any kind of element are joined together chemically. If a molecule contains two or more different elements, it is known as a compound. A water molecule is a compound of the elements hydrogen and oxygen.
If an atom or molecule becomes electrically charged by gaining or losing one or more electrons, it becomes an ion. If the atom gains electrons, it has a negative charge. If it loses electrons, it has a positive charge.
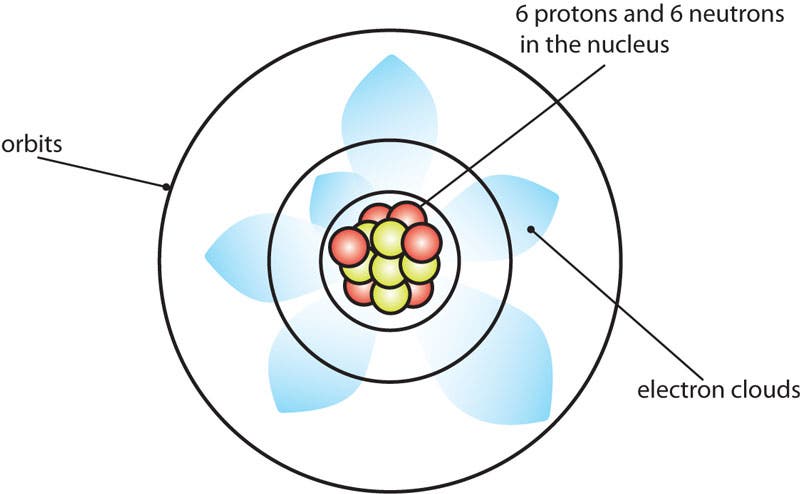
The Schrodinger Atom for Carbon
Erwin Schrodinger's model is a more correct representation. It includes electron clouds.
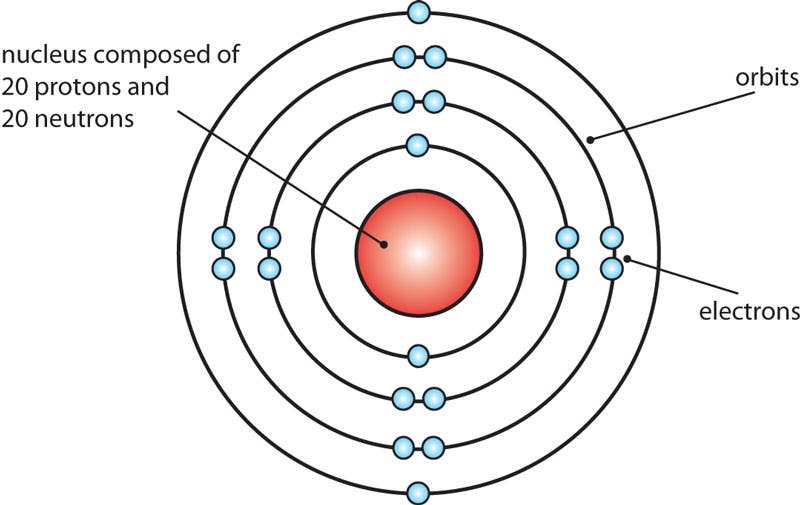
Bohr Model of Calcium
Bohr originally developed his atomic model to demonstrate the way in which electrons of hydrogen atoms changed orbits. Physicists today know that this version is not completely accurate, but it is still used because it offers a clear explanation of atomic structure at an introductory level.
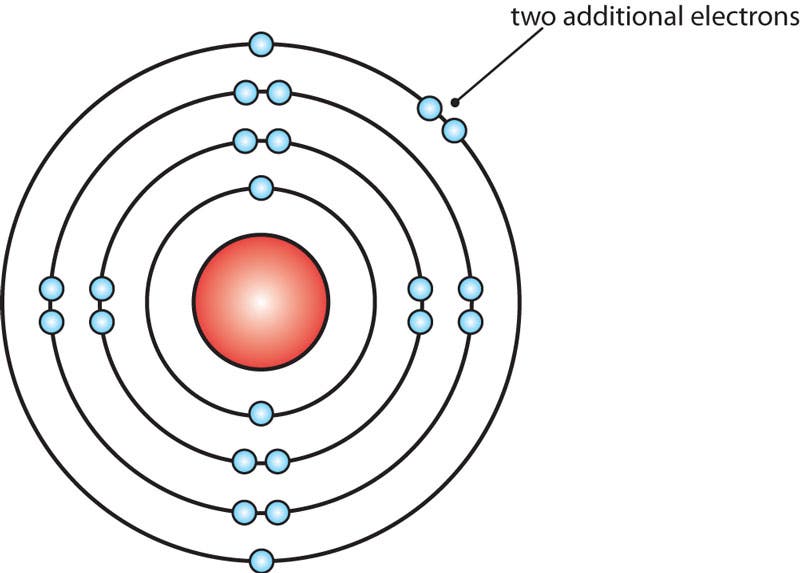
Bohr Model of a Calcium Ion
The calcium molecule above has gained two negatively charged electrons to become a calcium ion.

