What is an Isotope?
Isotopes
A chemical element may be represented by atoms with the same number of protons but a different number of neutrons. These variations are different isotopes of the same element. For example, the common isotope of hydrogen (1H1) has no neutrons, another isotope, deuterium (2H1), has one neutron, and a third one, tritium (3H1), has two. In the standard nomenclature the left superscript indicates the number of nucleons (protons and neutrons) and the right subscript the number of protons.
Heavy Water
Heavy water (2H2O) contains molecules of water (H2O) in which regular hydrogen has been replaced by its isotope deuterium. Hydrogen has a single proton as its nucleus, while deuterium has a proton and a neutron. Heavy water is used as a moderator (absorber of neutrons) in nuclear reactors.
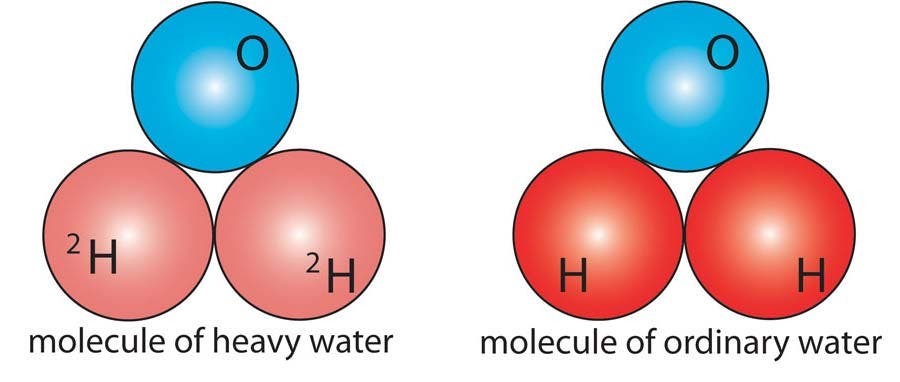
Heavy Water
Heavy water (2H2O) contains molecules of water (H2O) in which regular hydrogen has been replaced by its isotope deuterium.

